
A Review of Research on the Biological Transmutation of Chemical Elements
Based on an unfinished paper by Prof. L.W.J. Holleman
8. Experiment V
[This section covers the 5th experiment in Holleman's numbered series. The sources available for this section were the laboratory notebooks and various loose notes. Where relevant, details are given here of some of the other experiments and tests leading up to this experiment. An overview of them may be found in section 6.3.2].
8.1. Materials and Methods
8.1.1. Overview
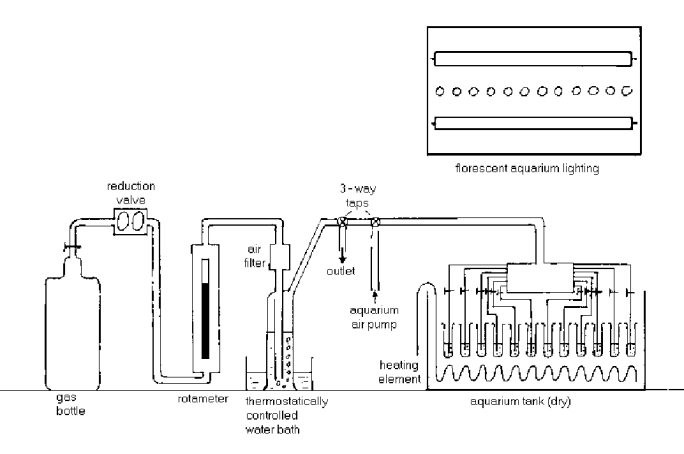
Figure 7.
The experimental set up is shown in figure 7. The gas supply was essentially the same as experiment II, but with a few minor improvements. A reduction valve and a rotameter provided extra control of the overall gas pressure. The filtered air passed into a gas wash bottle which was no longer used to grow an inoculation culture. The gas wash bottle was warmed in a thermostatically controlled water bath. This warming increased the water content of the gas which helped reduce evaporative losses in the culture tubes.
A three way tap enabled the gas system to be tested independently of the algal cultures. A second three way tap enabled a filtered air stream, supplied by an aquarium pump, to be switched into the system. This air stream was used, when needed, to aid the evaporation of the culture tube contents after growth. The inlet tubes, normally positioned near the bottom of the cultures, were raised above the liquid surface for this purpose.
After this second three way tap the gas stream was split 12 ways by
a distributor head. Each of the silicon rubber tubes exiting the
distributor head supplied the carbon dioxide enriched air to a rack
of up to twelve culture tubes. Each of these rubber supply tubes
possessed a pinch tap
which gave individual pressure control to
each test tube. The gas inlet tubes themselves were quartz glass
capillary tubes which loosely fitted through a hole in the plastic
test tube lid. The inlet tubes were held in place either side of
the lid by small, closely fitting rings of silicon rubber tubing.
The gas, which bubbled through the culture fluid, not only supplied
carbon dioxide gas, it also supplied the necessary agitation to
reduce the sinking and clumping of the algal cells, and thus
promoted homogeneous growth throughout the culture.
The experimental procedure was essentially the same as that outlined in section 7.1.1, etc.
The control cultures were different from experiment II. The inoculation procedure was identical to that of the experimental tubes. The controls were then heated for approximately 1.5 hours at 100 degrees to kill the algae. They were then (after cooling) replaced alongside the experimental tubes and were treated (including gas supply) identically to all other tubes, also including any additions of nitric acid.
8.1.2. Apparatus and Materials
8.1.2.1. Environmental Control
Because the previous shaking bath became unusable, an alternative method of maintaining the external environmental conditions conducive to maximal algal growth became necessary. A dry aquarium tank was chosen as the culture container. A thermostatically controlled heating element enabled the required temperature of 27 degrees inside the culture tubes to be maintained. The tubes themselves were held in a test tube rack which held 12 tubes in 2 rows of 6. Presumably the tank had a loosely fitting lid, though this was never recorded.
8.1.2.2. Containment Materials
The algal suspension was contained in quartz glass test tubes. Unfortunately the only further details given was that they were used to work with 5ml of liquid, and that there was 2 or more centimetres of space above that. The properties of these quartz tubes were presumably identical to those of the quartz Vitreosil dishes described in section 7.1.2.2.
8.1.2.3. Other Glassware
The only recorded changes in glassware since those described in section 7.1.2.3 were the quartz glass capillary tubes used as gas inlet tubes, to bubble air through the culture solutions.
8.1.2.4. Chemicals
Presumably the same chemicals were used as described in section 7.1.2.4. It is not clear whether the
Suprapure
nitric acid recorded in this section was the same
though. An examination of the published trace element impurities
apparently showed copper levels of 0.2x107M/l. In the
nutrient solution it was at a level of 1x107M/l. The
toxicity of the copper was given by Holleman to be at a level of
1.5x107M/l and thus could easily be attained during
normal experimental procedure. There was no evidence that Holleman
took any measures to further assess the situation, or to take
precautionary action.
8.1.2.5. Gas Supply
The essential details are given in section 8.1.1. The gas wash flask in this experiment again played a double role. This time, apart from helping to clean the gas, the water vapour picked up helped to compensate for evaporation in the culture tubes. The temperature of the thermostatically controlled water bath in which the flask sat was unfortunately only mentioned twice: once at 27 degrees when it was used for an earlier algal culture experiment; and once at 55 degrees when it was unsuccessfully used in a test to control the aforementioned evaporation problem of the cultures. At this latter temperature too much water vapour was carried over by the gas, leading to an increase in the volume of the cultures! I can only surmise that a temperature of 27 degrees was used, the same as that of the cultures. To reduce evaporation in the water bath itself, Holleman proposed using floating objects such as ping-pong balls.
Any gas leaks from poorly fitting connections were sealed using
Blue Tack
. The foaming which occurred in the tubes was due to the
bubbling of the gas through the cultures. It was successfully
prevented by smearing a very fine layer of Vaseline
over the
outsides of the capillary inlet tubes.
8.1.2.6. Lighting
The lighting used here was supplied by 2[?] fluorescent lights (PL9, 9W-600lumen, Pope) [aquarium lights?], one on each side of the test tube rack and at a distance from the tubes of 8-9cm. They were, however, acknowledged to be insufficient in intensity for maximum algal growth.
Whilst not explicitly stated, one may assume that the gas supply and lighting were left continuously on for the duration of an experiment (except when interrupted by a holiday or period in hospital).
8.1.2.7. Sterilisation
The measures taken were essentially the same as in section 7.1.2.7. The following steps were introduced for experiment IV and presumably (where relevant) hold true for Experiment V:
- Glass beakers sterile (inside).
- Labline shaking bath cleaned out with 70-alcohol.
- Ditto for Perspex lid.
- Cotton wool filters autoclave.
- Twice distilled water filtered by Millipore filter.
- Ditto nutrient solution.
- In Laminar Flow Cabinet:
- pipetting with sterile pipettes of sterile nutrient solution and sterile stem culture from Biophysics.
- -Tray of culture beakers brought over to shaking bath suitably covered.
8.1.3. Nutrient Solution
This remained the same as that described in section 7.1.3. The quantities of some of the individual elements present are given below:
| M/l | |
| K | 10x10-3 |
| Na | 7.3x10-3 |
| Ca | 0.1x10-3 |
| Mg | 0.1x10-3 |
| Phosphate | 5.0x10-3 |
| Sulphate | 0.9x10-3 |
| Chloride | 0.1x10-3 |
| Nitrate | 10 x10-3 |
8.1.4. Algal Cultures
8.1.4.1. Production of Cultures
This was essentially the same as in section 7.1.4.1, except that individual inoculation cultures were no longer grown by Holleman himself in the wash flask, but were obtained directly from Biophysics. They were stored in tap water cooled flasks at about 17 degrees in dim daylight until needed.
8.1.4.2. Measurement of Cell Number
For experiment V the Walker method of chlorophyll measurement was followed. It entailed the simple, direct measurement of the optical density of the (homogeneous - shaken -) culture at a wavelength of 600nm. Though normally undiluted, the recommended dilution of 1:26 was used for other tests and experiments when convenient.
For more accurate chlorophyll determination the van Hille method, described in section 7.1.4.2, was found to be preferable.
8.1.5. Procedure
8.1.5.1. Growth of Cultures
The procedure was basically the same as that described in section 7.1.5.1. The only difference was the volumes used: 5ml Kuhl (1/10Mg) inoculated by 0.2ml Chlorella. A fresh culture from Biophysics was used each time. All the tubes involved in the growth cycles were set up and subsequently ashed in pairs.
8.1.5.2. Ashing and Provision of New Nutrient Solutions
Some changes were made since experiment II (section 7.1.5.2). Firstly, after growth was completed (14 days) the gas supply was stopped and the supply tubes disconnected. The tap for the aquarium air pump was connected and the tubes transferred to the Aluminium Heating Block which was at approximately 100 degrees. The air was pumped over the surface of the culture medium (distance about 2cm) in the tubes. This enabled evaporation to take ca. 1.5 hours. The tubes were then placed in the furnace for 1 hour and heated to 500 degrees: The time spent at 500 degrees (or slightly above) was 10 minutes. After cooling each tube received 2ml twice distilled water and the drying and ashing procedure was repeated such that all carbon particles were fully burnt and driven off (this process was repeated a third time if necessary). The tubes then received 3ml of water and 2ml of 0.01N nitric acid to neutralise the newly made up nutrient solutions ready for the next cycle.
8.1.5.3. Controls
The first control pair of tubes for experiment V were identical to
the growth cultures except that they developed (as near as
possible) in total darkness. This was for the first cycle of the 6
cycles through which it passed. All other subsequent control tube
pairs, as well as the first control pair (in all other subsequent
cycles), were heated in the aluminium block heater at 100 degrees
for 1.5 hours to kill the algae present (it was not stated to what
extent evaporation had taken place during this heating). The
experimental growth cycle was only started once the controls were
placed alongside the experimental growth pairs. The timing of when
the controls and their complimentary experimental pairs were
grown
with regards to the cycle number are easiest given in the
form of a table:
|
\cycles number\ |
1 | 2 | 3 | 4 | 5 | 6 | 7 |
| 1 | B + N | B + N | B + N | B + N | B + N | B + N* / | Cc + Rc* |
| 2 | C + R | C + R | C + R | C + R | C + R / | Dc + Qc | Dc + Qc* |
| 3 | D + Q | D + Q | D + Q | D + Q / | Ec + Tc | Ec + Tc | Ec + Tc* |
| 4 | E + T | E + T | E + T / | Fc + Sc | Fc + Sc | Fc + Sc | Fc + Sc* |
| 5 | F + S | F + S / | Vc + Wc | Vc + Wc | Vc + Wc | Vc + Wc | Vc + Wc* |
| 6 | V + W* / | Ac + Mc | Ac + Mc | Ac + Mc | Ac + Mc | Ac + Mc* | [Ac + Mc*] |
|
|
[Ac + Mc] |
|
|||||
| N - growth tube; Nc - control; Nc* - control to be ashed. | |||||||
8.1.5.4. Chemical Analysis
This differed from that described in section 7.1.5.4 on a number of different points. The ashes for analysis were dissolved up in 4ml of distilled water to which 1ml 1.000N HCl was added. This was warmed for 1.5 hours in the aluminium block at ca.100 degrees. The Kalignost method for potassium analysis was not used. Potassium and sodium, analysed by flame photometry, were the only two elements examined in experiment V.
8.2. Results
8.2.1. Analytical Results
| Number of cycles | Potassium | Sodium | ||||||||
|
Cali- bration solution |
Experiment | Control |
Cali- bration solution |
Experiment | Control | |||||
| Tube | Result | Tube | Result | Tube | Result | Tube | Result | |||
| 1 | 50 | V | 80 | Cc | 72.5 | 20 | V | 33.5 | Cc | <31 |
| W | 79.5 | Rc | 79 | W | 34 | Rc | 34 | |||
| 2 | 50 | F | 82 | Dc | 74 | 20 | F | 35 | Dc | 31.5 |
| S | 83 | Qc | 82 | S | 35 | Qc | 35 | |||
| 3 | 50 | E | 83 | Ec | 81.5 | 20 | E | 35.5 | Ec | 35 |
| T | 84 | Tc | 82 | T | 36 | Tc | 35 | |||
| 4 | 50 | D | 86 | Fc | 83 | 20 | D | 37 | Fc | 35 |
| Q | 84 | Sc | 88.5 | Q | 36.5 | Sc | 36.5 | |||
| 5 | 50 | C | 91 | Vc | 90 | 20 | C | 37 | Vc | 37 |
| R | 87 | Wc | 87 | R | 36.5 | Wc | 36.5 | |||
| 6 | 40 | B | 94.5 | Ac | 93.5 | 40 | B | 51 | Ac | 48 |
| N | 98 | Mc | 74.5 | N | 49 | Mc | 45.5 | |||
As before (see section 7.2.1), the ratio of Potassium:Sodium was calculated to help compensate for evaporative and other losses:
| Cycle number | Growth | Control | ||||
| Tube | Result | Average | Tube | Result | Average | |
| 1 | V | 2.39 | 2.37 | Cc | >2.34 | 2.33 |
| W | 2.34 | Rc | 2.32 | |||
| 2 | F | 2.34 | 2.36 | Dc | 2.26 | 2.30 |
| S | 2.37 | Qc | 2.34 | |||
| 3 | E | 2.34 | 2.34 | Ec | 2.33 | 2.34 |
| T | 2.35 | Tc | 2.34 | |||
| 4 | D | 2.32 | 2.31 | Fc | 2.37 | 2.40 |
| Q | 2.30 | Sc | 2.42 | |||
| 5 | C | 2.46 | 2.42 | Vc | 2.43 | 2.40 |
| R | 2.38 | Wc | 2.38 | |||
| [6 | 1.85 | 1.92 | Ac | 1.95 | 1.80] | |
| N | 2.00 | Mc | 1.64 | |||
| Experimental cycles 1 - 5: | Control average | 2.354 | ||||
| Growth average: | 2.354 | |||||
No significant difference in the potassium content was found between experimental and control cultures. This was a very different result to that of experiment II.
The potassium content of 5 of the inoculation solutions, that were at that time still available (it was 14 months since the first culture was obtained), were measured and all were in approximate in agreement. The potassium contents of four Kuhl solutions were measured; two were of 1/10Mg; the other two 1/1Mg; one of each were hydrolysed as normal, the other two were not hydrolysed [this treatment is not clear; I assume that this latter pair were neither dried nor ashed, i.e. that they were used without any treatment whatsoever]. The potassium concentrations of the 4 Kuhl solutions were in good agreement, but possessed significantly lower values than those of the cultures. All five algal cultures had been stored for 4-14 months in a variety of polythene and glass containers at a number of different temperatures and lighting levels. The results are given below:
| Sample | Result (M/l) |
| Bf3 | 87.5 |
| Bf4 | 91 |
| Bf7(K) | 80 |
| Bf6 | 91 |
| Bf7 | 84 |
|
|
|
| 1/10 with | 75 |
| 1/1 with | 72 |
|
|
|
| 1/10 without | 74 |
| 1/1 without | 71 |
See section 10.2.1 for a discussion of these results.
8.2.2. Health of the Cultures
Varying degrees of clumping and sinking occurred in all tubes. Development in general was found to be variable.